The following article is from the Stroke Horizon, by the CASSISS research group.
In the early morning of August 10, 2022, Beijing time, led by the team of Professor Jiao Liqun from the Department of Neurosurgery of Xuanwu Hospital of Capital Medical University, a multi-center, randomized controlled clinical trial jointly conducted by 8 hospitals in China - CASSISS research results were published online in top international medical journals "JAMA" (IF 157.335). The 10-year CASSISS study has provided new medical evidence internationally for endovascular treatment of intracranial artery stenosis. The CASSISS study compared the effects of stenting combined with medical therapy versus medical therapy alone on stroke and death in patients with symptomatic intracranial artery stenosis. The results showed that in patients with symptomatic, severe intracranial arterial stenosis, stenting combined with medical therapy was comparable to medical therapy alone in preventing stroke or death.
Background
Stroke is the leading cause of death in my country. Intracranial atherosclerotic stenosis is the most common cause of ischemic stroke in Asian populations, with a rate as high as 46.6%. In Western countries, the proportion is only 10%-15%. Therefore, intracranial atherosclerotic stenosis is one of the most common causes of ischemic stroke in China, posing a huge challenge to our prevention and treatment of stroke.
Endovascular therapy such as interventional stenting has been regarded as a potential treatment for intracranial atherosclerotic stenosis. The SAMMPRIS and VISSIT studies in the United States are the only two multicenter randomized controlled studies in the world comparing stents with drug therapy alone, and they were mainly completed in Western populations. The results show that stent treatment has higher complications and is worse than medical treatment alone; however, there have always been many doubts in the academic community for these two studies.
In recent years, multiple prospective registration studies and real-world studies at home and abroad have shown that intracranial artery stents are still safe, and the risk of complications is only 2.0%-4.3%, which is in sharp contrast with the 14.7% of the SAMMPRIS study. Therefore, the treatment strategy of intracranial atherosclerotic stenosis remains controversial.
In view of the fact that the SAMMPRIS study is based on Caucasians in the United States and the shortcomings of its own trial design, the era urgently needs Chinese data, with the help of the efforts and contributions of Chinese doctors to verify the safety and efficacy of endovascular therapy in Chinese data. Therefore, with the support of the "Twelfth Five-Year" National Science and Technology Support Program, CASSISS research was born in response to the times.
The CASSISS study (China Angioplasty and Stenting in the Treatment of Symptomatic Severe Intracranial Arterial Stenosis: A Multicenter, Randomized Controlled Clinical Trial) is the third in the world and the first in China and Asia. Led by the team of Professor Jiao Liqun from Xuanwu Hospital of Capital Medical University and National Medical Center for Neurological Diseases, together with Professor Wang Daming of Beijing Hospital, Professor Shi Huaizhang of the First Affiliated Hospital of Harbin Medical University, Professor Cai Yiling of the Special Medical Center of Strategic Support Forces, Professor Wu Wei of Qilu Hospital of Shandong University, The team of Professor Li Tianxiao of Henan Provincial People's Hospital, Professor Zhao Zhenwei of Tangdu Hospital of Air Force Military Medical University, and Professor He Weiwen of the Second Affiliated Hospital of Guangzhou Medical University jointly completed the optimization of patient selection criteria, operation timing, medical center capacity, etc. Effect of drug therapy on stroke or death in patients with symptomatic, severe intracranial atherosclerotic stenosis. After nearly ten years of concerted and unremitting efforts of all researchers, the research results were finally published in the top international medical journal "JAMA".
Method
In response to the design flaws of the SAMMPRIS study, the CASSISS study proposes two important improvements:
1. Select more experienced doctors and more rigorous screening of patients, reducing 30-day complications (safety);
2. The follow-up period was extended to 3 years, and the long-term protection (efficacy) of endovascular therapy on intracranial artery stenosis was observed.
Therefore, the CASSISS study was designed to re-evaluate the safety and efficacy of endovascular therapy for intracranial artery stenosis, and to provide Chinese evidence for the treatment of intracranial artery stenosis internationally.
The CASSISS study innovatively set up a two-phase trial: a pilot phase I trial and a phase II randomized controlled trial.
Pilot test phase:
A prospective, single-arm observational study. From July 2013 to March 2014, according to the inclusion and exclusion criteria of follow-up randomized controlled trials, 100 patients were consecutively enrolled for stent treatment, to evaluate the risk of perioperative surgery, to ensure the safety of subjects and the qualification of participating centers, and to verify the follow-up RCTs Test operability. The results showed that the 30-day stroke or death rate after stenting in this group of patients was 2.0%, which was much lower than the results of the SAMMPRIS and VISSIT studies. The pilot test verified the operability of the follow-up RCT test, and through this phase of the study, the research center was compressed from 13 to 8 more qualified.
Randomized controlled trial phase:
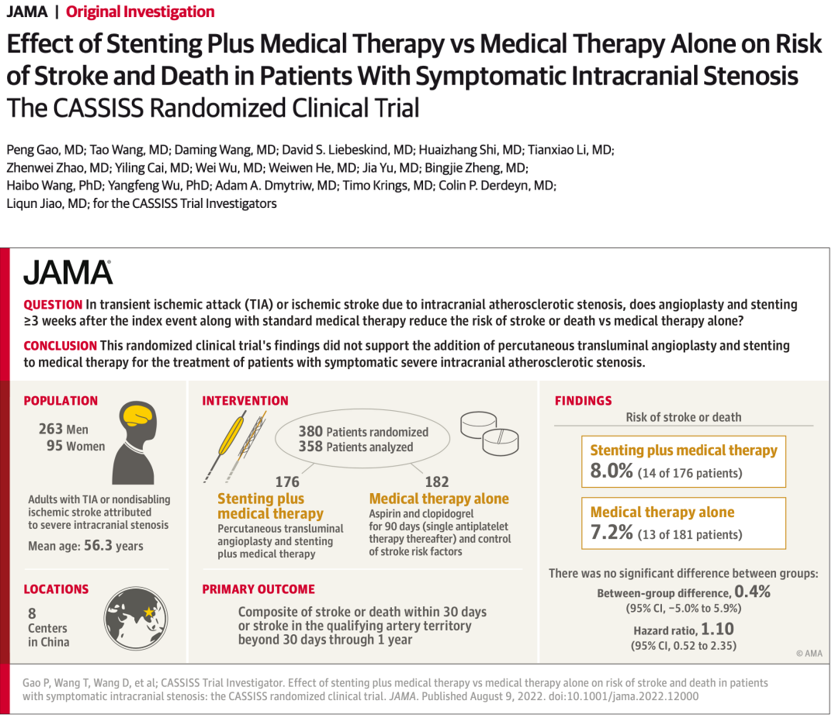
A multicenter, open-label, blinded-assessed randomized controlled clinical trial. From March 2014 to November 2016, from 8 medical centers, 380 patients with ischemic events over 3 weeks after onset, manifesting as TIA or non-disabling, non-perforator ischemic stroke, with severe stenosis (70%-99%) of patients with intracranial atherosclerotic stenosis. According to the ratio of 1:1, they were randomly divided into two groups: stent combined with drug therapy and drug therapy alone. The primary outcomes were stroke or death within 30 days and stroke in the vascular region of responsibility from 30 days to 1 year, and secondary outcomes included stroke or death in the vascular region of responsibility at 2 or 3 years. The follow-up period was 3 years, and the follow-up of the last patient ended on November 10, 2019.
Result
Of the 380 patients, 358 patients were finally confirmed eligible for enrollment and completed the trial, including 176 in the stent group and 181 in the drug group.
The primary outcome showed that there was no statistically significant difference between stent and medical therapy compared with medical therapy alone (8.0% [14/176] vs. 7.2% [13/181]; hazard ratio: 1.10, 95%CI 0.52-2.35 ; P=0.82). None of the 5 secondary outcomes showed statistically significant differences. For example, 3-year responsible vessel area stroke (11.3% [19/168] vs. 11.2% [19/170]; hazard ratio 1.00, 95% CI 0.53-1.90; P=1.00). The 3-year mortality rates were 4.4% [7/160] and 1.3% [2/159], respectively (hazard ratio, 3.75 [95% CI 0.77-18.13]; P = 0.08).
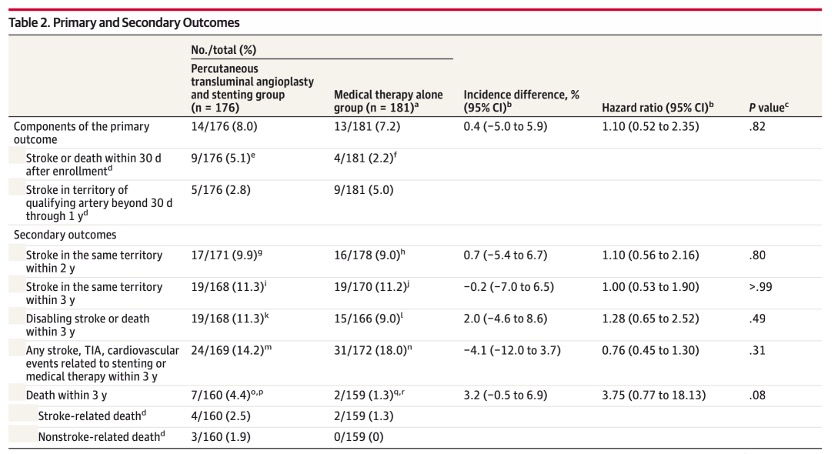
primary and secondary endings
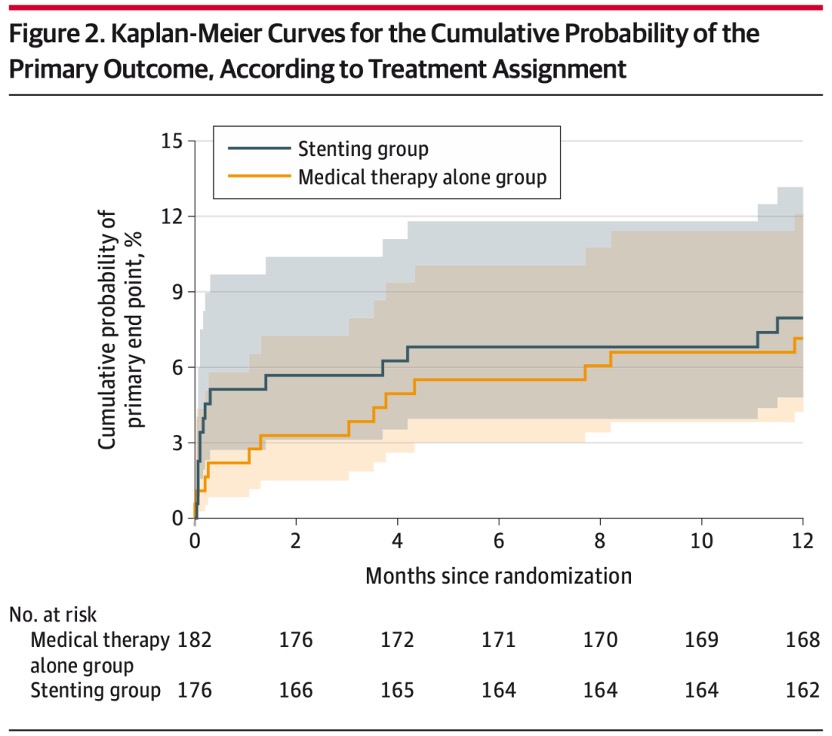
K-M curves for the primary outcome
In conclusion
For patients with TIA or ischemic stroke caused by symptomatic intracranial atherosclerotic stenosis, the risk of stroke or death within 30 days and the risk of stroke or death within 30 days and 30-day to There was no significant difference in the risk of stroke in the responsible vessel region at 1 year. (The CASSISS study is supported by the National Science and Technology Support Program of the “Twelfth Five-Year Plan”, the project number is 2011BAI08B04, and the ClinicalTrials.gov registration number is NCT01763320)
With high-quality research data and high-level evidence, the CASSISS study has resolved two decades of controversy in this field, and may become the "final work" of clinical guidelines in this field. The important conclusions are:
1. Experienced Chinese doctors can obtain significantly better safety than U.S. studies, and efficacy not inferior to drug therapy;
2. Under the current diagnostic evaluation system for intracranial atherosclerotic stenosis, interventional stent therapy has no additional benefit to patients compared with medical therapy alone.
The CASSISS study also provides important implications for the future research direction of intracranial atherosclerotic stenosis. Future research will focus on conceptual and technological innovations in disease diagnosis and assessment, as well as advances in interventional devices and technologies.
Contributing Authors:
Gao Peng
Deputy Chief Physician, Doctor of Medicine.
Deputy Chief Physician of Neurosurgery, Doctor of Medicine, Beijing Science and Technology Rising Star, and a public student sent by the Ministry of Education.
Deputy director of the Youth Committee of the Interventional Medicine Branch of the Beijing Medical Association, member and secretary of the Neurointerventional Group of the Interventional Medicine Branch of the Beijing Medical Association, and member of the Youth Committee of the Neurointerventional Professional Committee of the Chinese Medical Association.
Has been committed to the clinical diagnosis and treatment of ischemic cerebrovascular disease. From 2007 to 2009, he studied at the Institute of Cerebrovascular Diseases, University of California, San Francisco. Published 12 SCI papers as the first author, and participated in the editing of one of the refereed works. Presided over one of the National Natural Science Foundation of China Youth Fund and the Beijing Science and Technology Commission Science and Technology Rising Star Program. Participated in the national "Twelfth Five-Year" scientific and technological support plan project "Research on the minimally invasive technology system for cerebral revascularization in ischemic cerebrovascular disease" and the "Thirteenth Five-Year" national key scientific and technological support plan "Digital cerebral blood flow reserve function diagnosis and evaluation technology and Its Application Research".
Wang Tao
Attending Physician, Doctor of Medicine.
In 2017, he graduated from the Peking Union Medical College (Tsinghua University School of Medicine) with an eight-year clinical medicine program. Committed to the surgical and interventional diagnosis and treatment of ischemic cerebrovascular diseases such as intracranial artery stenosis, carotid artery stenosis and moyamoya disease. So far, he has published 20 SCI papers and 1 Chinese core journal paper as the first author or co-first author. Participated in 2 books. Participated in the "13th Five-Year" National Key R&D Program, Beijing Municipal Science and Technology Commission and other provincial and ministerial-level projects, and presided over 3 projects including the Beijing Medical Management Center Cultivation Program. He has a deep understanding of data mining and information analysis in the clinical context, and is good at clinical research design and systematic review methods. Successful registration of multiple Cochrane systematic reviews investigating the diagnosis, assessment and treatment of intracranial and carotid stenosis.
Wang Daming
Chief physician, professor, doctoral tutor, member of the National Committee of the Chinese People's Political Consultative Conference.
He is currently the chief expert of neurosurgery in Beijing Hospital. He is also a member of the Chinese Society of Neurosurgery and the deputy director of the Beijing Neurosurgery Branch. Under the tutelage of Professor Ling Feng, a famous Chinese neurointerventional expert, and Professor Luc PICARD, President of the World Federation of Interventional Neuroradiology. Specializes in the interventional treatment of cerebral and spinal vascular diseases. The first work carried out or reported in domestic or international are: establishment of cerebral arteriovenous malformation animal model, hemodynamic model and embolization research, electrolytic detachable coil embolization for intracranial aneurysm, embolization standard of intracranial aneurysm Special research on three-dimensional cerebral angiography in the diagnosis and treatment of intracranial aneurysms, stent placement for intracranial artery stenosis, endovascular dilation and stent placement for carotid and vertebral artery stenosis under cerebral protection devices, etc. .
David S. Liebeskind, M.D.
Professor of Neurology at the University of California, Los Angeles (UCLA), Director of the UCLA Stroke Center, Director of the Neurovascular Imaging Research Core, leading global efforts to advance data science and precision medicine of stroke imaging for prevention, acute therapies and recovery after stroke.
Director of the UCLA Cerebral Blood Flow Laboratory, Director of Outpatient Stroke and Neurovascular Programs and Director of the UCLA Vascular Neurology Residency Program, training the next generation of vascular neurologists and stroke experts.
Corresponding Author
Jiao Liqun
Chief Physician, Professor.
Director of Interventional Radiology Department, Xuanwu Hospital, Capital Medical University, Deputy Director of Neurosurgery, Director of Cerebral Revascularization Center, Doctoral Supervisor.
Vice-Chairman of the Neurointerventional Committee of the Chinese Medical Doctor Association; Chairman of the Neurointerventional Committee of the National Health and Health Commission's Education Center; Vice-chairman of Ischemic Stroke Intervention Special Committee; editor-in-chief of "Chinese Journal of Cerebrovascular Diseases"; central health care consultation expert; vice-chairman of Beijing Interventional Medicine Association.
Graduated from Shandong Medical University, successively studied under Professor Zhu Shugan and Professor Ling Feng, obtained a master's degree and a doctorate degree in neurosurgery, and completed postdoctoral research at Peking University under the guidance of Professor Bao Shengde.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
A single copy of these materials may be reprinted for noncommercial personal use only. "China-INI," "chinaini.org" are trademarks of China International Neuroscience Institute.
© 2008-2021 China International Neuroscience Institute (China-INI). All rights reserved.